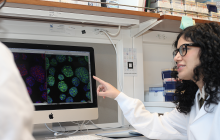
Patient Derived Prostate Cancer Organoids to Advance Precision Medicine
June 28, 2018
A small amount of tumor cells taken from a patient are embedded in an extracellular matrix and grown in the laboratory. As the cells develop in vitro, they form three-dimensional structures that mimic the patient’s disease. These structures, called tumor organoids, are at the forefront of cancer research – they allow researchers to test new drugs and investigate new treatments with a personalized, targeted approach.

Spotlight on Dr. Jan Krumsiek
May 8, 2018
Welcome, Dr. Jan Krumsiek, Assistant Professor of Physiology and Biophysics in the Department of Physiology and Biophysics, and the newest member of the Englander Institute for Precision Medicine. Previously a visiting fellow in the Leandro Cerchietti lab, Dr. Krumsiek answered a few quick questions to elaborate on his experience and interests.
Please provide a brief overview of your work before Weill Cornell Medicine.

Hyperion Module added to EIPM Arsenal
March 30, 2018
At the Englander Institute for Precision Medicine (EIPM) we strive to be at the forefront of translational research. In conjunction with the WorldQuant Initiative for Quantitative Prediction, we are delighted to announce our latest technological gem: a CyTOF coupled to the Hyperion module – one of only 30 of its kind currently in use and the only one in the NYC.

Voronoi Selection for Drug Network Visualization in MR
March 12, 2018
Researchers at the Englander Institute for Precision Medicine (EIPM) bring together basic science, medicine, DNA sequencing and big data to deliver the next generation of precision medicine; the most promising approach to tackle diseases that have thus far eluded effective treatments like cancer, neurodegenerative diseases, and rare genetic conditions.
A New Regulatory Threat to Cancer Patients
February 26, 2018
25 February 2018
By Dr. Olivier Elemento
"The federal government is threatening to limit treatment options for doctors fighting cancer. A regulatory decision due this Wednesday from the Centers for Medicare and Medicaid Services (CMS) could undermine the care delivered to the more than 1.6 million Americans who are diagnosed with cancer each year.
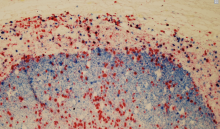
AI Aids in Cancer Diagnosis
February 12, 2018
An artificial intelligence (AI) program developed by Weill Cornell Medicine and NewYork-Presbyterian researchers can distinguish types of cancer from images of cells with almost 100 percent accuracy, according to a new study. This new technology has the potential to augment cancer diagnosis techniques that currently require the human eye.

What is CH?
January 13, 2018
What is hematopoiesis?
Hematopoiesis refers to the body’s biological process of making blood. Our bone marrow contains blood-forming cells termed “hematopoietic stem cells.” These stem cells produce all the blood cells in our body.
What is clonal hematopoiesis (CH)?
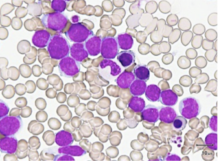
Signs of AML may be present years before diagnosis
December 13, 2017
Patients with acute myeloid leukemia (AML) may have genetic mutations in their blood indicating they are at high risk of developing the disease about nine years before diagnosis, according to research from Weill Cornell Medicine and NewYork-Presbyterian investigators. They presented their findings at the 59th annual American Society of Hematology meeting Dec. 10 in Atlanta.

Tumor Models
November 10, 2017
The Englander Institute for Precision Medicine (EIPM) has developed unique models to study cancer in its laboratories, such as growing mini tumors called ‘organoids’.
Human cancer tissue that is grown into organoids in the laboratory could be used to test drug responses and to personalize therapy.
Organoids can be made to resemble organs or tissues such as gut, kidney, pancreas, liver, breast, prostate, and even brain tissue, all complete with accurate micro-anatomy.

Targeting Cancer
November 10, 2017
Precision medicine harnesses a unique mix of personal genetic, genomic and clinical information to inform patients’ medical care, for both treatment and prevention of diseases. This information includes individual genomic DNA sequences, which can potentially identify variants that cause disease, and in some cases predict how a patient will respond to a particular drug.

